- About DPH
- Our Services
- Our Programs
- Healthy Living
- Records, Permits & Licensing
- Knowledge Sharing & Collaboration
- Diseases & Conditions
- Training
Helpful Links
CA Food Code (pdf)
FDA Form 3757 -- voluntary registration (pdf)
Menu Labeling FAQ (pdf)
Vending Machines FAQ (pdf)
Nutrient Verification Form (pdf)
Program Contact
Tiffany Yim
(415) 252-3899
Tiffany.Yim@sfdph.org
Nutrition Labeling Requirements for San Francisco City & County Chain Food Establishments and Vending Machines
Signed into law on March 23, 2010, the Patient Protection and Affordable Care Act requires restaurants with 20 or more locations nationally to add calorie counts to menus, menu boards and drive-thru menu boards for standard menu items. The law also requires that chain restaurants provide additional nutritional information upon request. Furthermore, vending machine operators who own or operate 20 or more vending machines must disclose calorie content for certain items. Food service establishments that do not meet this quantitative threshold may voluntarily register to be subject to the menu labeling requirements.
In 2015 the US Food and Drug Administration (FDA) finalized regulations for the implementation of the menu and vending machine labeling requirements. The FDA extended the compliance deadline to May 7, 2018 for menu labeling and July 26, 2018 for vending machines.
Frequently Asked Questions about the Nutrition Labeling Legislation
What businesses are covered by the final rule?
To be covered by the final rule, an establishment must meet certain criteria. First, the establishment must be a restaurant or similar retail food establishment. Next, the establishment must: (1) be part of a chain of 20 or more locations, (2) doing business under the same name, and (3) offering for sale substantially the same menu items.
Establishments, such as restaurants that are quick service and/or sit-down, food take-out facilities, pizza delivery establishments, food facilities in entertainment venues (e.g., movie theaters, bowling alleys), cafeterias, coffee shops, superstores, and grocery and convenience stores, are covered if they meet the criteria listed above.
What are the requirements of the menu labeling legislation?
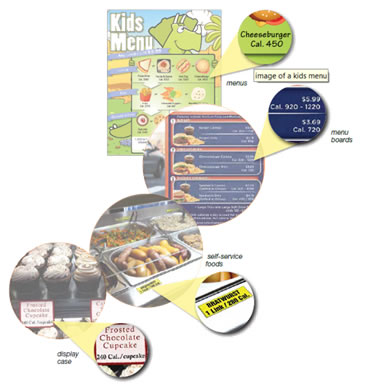
Covered restaurants and similar retail food establishments are now required to: (1) disclose calorie information on menus and menu boards for standard menu items; (2) post a succinct statement concerning suggested daily caloric intake on menus and menu boards; and (3) post a statement that "additional nutrition information is available upon request" on menus and menu boards.
What nutrition information is required and where should it be posted?
The rule requires establishments to provide the following written nutrition information for standard menu items to consumers upon request: total calories, calories from fat, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrates, fiber, sugars, and protein. In addition, calorie information must be declared on signs adjacent to foods on display and self-serve foods that are standard menu items. The written nutrition information can be provided on posters, tray liners, signs, counter cards, handouts, booklets, computers, or kiosks.
How is nutrition labeling useful to customers?
Approximately one third of the calories Americans eat and drink come from foods and beverages consumed away from the home. In addition, research shows that when we dine out we often eat less nutritious foods and underestimate the number of calories we are consuming.
People can clearly benefit by having access to additional nutrition information, and the new FDA rules will help to do just that. They are not requirements about what people should eat or what restaurants should serve, but rather they will place more information in the customer's hands so that they can make more informed choices for themselves and their families.
How does a business conduct nutritional analysis of its standard menu items?
Nutrient content declarations can be based on information obtained from nutrient databases, cookbooks, laboratory analyses, the Nutrition Facts Label on packaged foods, and other reasonable means.
Where can I found more information about the menu labeling legislation?
For industry questions about compliance with FDA regulations, contact:
- Telephone Hotline: 1-888-SAFEFOOD (1-888-723-3366)
- Email: industry@fda.gov
- Written Inquiry:
- Center for Food Safety and Applied Nutrition (HFS-009)
- Food and Drug Administration
- 5100 Paint Branch Pkwy
- College Park, MD 20740
For consumer questions about menu labeling, email: CalorieLabeling@fda.hhs.gov
Frequently Asked Questions about the Vending Machine Labeling Legislation
How do I know if my vending machine is covered by the final rule?
To be covered by the final rule, a person or entity must control or direct the function of 20 or more vending machines, which includes deciding which articles of food are sold or the placement of those items within the vending machine and who is compensated for the control or direction of the vending machine. Operators that do not meet the aforementioned threshold may choose to voluntarily register with the FDA to be covered by the rule.
What types of vending machine are covered?
By definition, a vending machine is "a self-service machine that, upon insertion of a coin, paper currency, token, card, or key, or by optional manual operation, dispenses servings of food in bulk or in packages, or prepared by the machine, without the necessity of replenishing the machine between each vending operation." In general, a vending machine could encompass—but not be limited to—those that sell soft drinks, packaged snacks, hot-and-cold cup beverages, refrigerated prepared food, and handfuls of nuts or candies. Game machines are not covered, even if they sometimes dispense candy or other edible items as part of the game.
Where does the calorie information have to be posted?
Calorie declarations can be placed on a sign close to the article of food or selection button (i.e., in, on, or adjacent to the vending machine). The sign does not necessarily need to be attached to the vending machine as long as the calorie declaration is visible at the same time as the food, its name, price, selection button, or selection number is visible. The sign must provide calorie declarations for articles of food that are sold from that particular vending machine. The rule also permits electronic or digital displays of the calorie information.
What information am I required to submit in order to voluntarily register with the FDA?
An operator (or its authorized official) must provide the FDA with the following information using Form FDA 3757 – Menu/Vending Labeling Registration Form.
For Restaurants and Similar Retail Food Establishments:
- Contact information (including name, address, phone number, and email address) for the authorized official;
- Contact information (including name, address, phone number, and email address) of each restaurant or retail food establishment being registered;
- All trade names the restaurant or similar retail food establishment uses;
- Preferred mailing address (if different from location address for each establishment) for purposes of receiving correspondence; and
- Certification that the information submitted is true and accurate; that the person or firm submitting the information is authorized to do so, and that each registered vending machine will be subject to the requirements of section 403(q)(5)(H) of the FD&C Act.
For Vending Machines:
- Contact information (including name, address, phone number, and email address) for the operator;
- Location address of each vending machine owned or operated by the vending machine operator that is being registered;
- Preferred mailing address (if different from the vending machine operator address) for purposes of receiving correspondence; and
- Certification that the information submitted is true and accurate; that the person or firm submitting the information is authorized to do so, and that each registered vending machine will be subject to the requirements of section 403(q)(5)(H) of the FD&C Act and 21 CFR 101.8.
Completed forms should be emailed to menulawregistration@fda.hhs.gov. If email is not available, you can either complete the fillable form and print it, or print a blank form and fill in the information by hand or typewriter; then:
- Fax to 301-436-2804 or
- Mail it to:
- FDA
- CFSAN Menu and Vending Machine Registration
- White Oak Building 22, Room 0209
- 10903 New Hampshire Avenue
- Silver Spring, MD 20993